Table of Contents
ToggleCHAPTER THREE: METHODOLOGY
Methodology is the longest and most examinable, take note.
3.1 Introduction
3.2 Study Design and rationale
3.3 Study setting and rationale
3.4 Study Population
3.4.1 Sample Size Determination
3.4.2 Sampling Procedure
3.4.3 Inclusion Criteria
3.5 Definition of Variables
3.6 Research Instruments
3.7 Data collection Procedure
3.7.1 Data management
3.7.2 Data analysis
3.8 Ethical Consideration
3.9 Limitations of the study
3.10 Dissemination of Results

Methodology therefore consists or covers the methods the researcher is to follow while carrying out research. Therefore it includes the following.
- Introduction.
- Study Design.
- Study Setting.
- Study Population.
- Sample Size. Determination.
- Diploma level studies should have a minimum of 30 participants.
- Student should give justification for selected sample size.
- Sampling Procedure.
- Inclusion Criteria.
- Definitions of Variables.
- Research Instruments.
- Data Collection Procedures.
- Data Management.
- Data Analysis.
- Protection of Human Subjects.
- Dissemination of Results.
- Limitations of the Study.
3.2 Study Design
Study or Research design defines the approaches, methods and the rationale of picking that appropriate research design
- Eg descriptive cross sectional design
- Approaches can be Quantitative/qualitative or both
- Note that it is advisable to use one of these at our level.
The design is the structure of the study. This is the framework for the methodology to be applied while collecting data, sampling, analyzing data, etc.
- The function of a study or research design is to ensure that the evidence obtained enables us to answer the initial question as unambiguously as possible. In other words, when designing research we need to ask: 1. Given this research question/problem, what type of evidence is needed to answer the question in convincing way?
- You should always state the reason/rationale for using that particular design (why that particular design).
Example: “The study will use a retrospective comparative study design and this is due to the fact that it involves comparing virological outcomes in 4 different groups of patients on different arms of first-line ART regimens. The study will also employ a quantitative method of data collection in order to quantify the most efficacious
1st– line ART regimen in terms of virological suppression”
3.3 Study Setting
- Also called the study area.
- It helps the reader to locate where your study is to be done from.
- Direct the reader in terms of location (Where are you’re
- going to do the study from?)
- Why that setting? (State the rationale for using that
- setting).
Example: “Study will be carried out at ART clinic of Kayunga Hospital in Kayunga district which is located in central part of Uganda. ART clinic operates on daily basis from Monday to Friday from 8am to 4pm. It has a total of 10 nurses, 2 laboratory technicians, 2 clinical officers and I medical officer. This clinic receives on average a number of 150 patients on every clinic day. The study setting was chosen because ART clinic serves a big population of about 4500 HIV/AIDS infected people”.
3.4 Study Population
- Explain the population from which your sample will be collected from.
- This is the population that the results will be generalised to.
- Give the rationale for the selected population.
Population: This is the total of items or events in a set; with relevant characteristics that a researcher need (It is the total number of potential subjects /respondents for a study).
The population should be clearly defined before a decision is taken on how to sample it.
• Sampling is not necessary if the population is small.
Example: “This study will be carried out among HIV-infected clients attending Kayunga ART clinic and who are on first-line ART regimens for at least three years. Kayunga ART clinic has a total of 4791 of which 2728 are on 1st line ART regimen. The clinic usually receives about 50 clients who are on 1st-line ART regimen per day and therefore a total of 250 clients on 1st-line ART will be available for Data collection within 5 days of data collection”.
3.4.1 Sample Size Determination
- Sampling is the process of selecting a subset(sample) from a large group of people(population)
- Steps in sampling
- Define the population
- Identify the sampling frame ie list of participants from which a sample can be selected
- Select a sampling procedure this could be probability or nonprobability sampling
- Determine the sample
- Draw the sample
- Give justification
- State the standardized method you will use to estimate
the sample size.
For example: “Using Krejcie and Morgan (1970)’s table, when a population is-250, a total of sample size of 150 respondent is supposed to be sampled”

3.4.2 Sampling Procedure
- This refers to the way you select your participants to include in your study.
- It can be Probability or non probability sampling.
- Probability sampling involves;
- Simple random sampling.
- Systemic sampling.
- Stratified sampling.
- Cluster sampling.
- Non probability sampling involves;
- Convenience sampling.
- purposive/ judgemental sampling.
- Snowball sampling.
- Quota sampling.
Explain how the subjects will be selected during sampling.
For example, a proportionate quota sampling method will be used to sample representative clients on the different first -line ART regimens.
- State the reason (rationale) why you have decided to use that particular procedure.
3.4.3 Inclusion Criteria
- This gives a narration of which people among the selected population will qualify to participate in your study.
- Those who do not qualify are the excluded from your study.
Inclusion criteria: are characteristics that the prospective subjects must have if they are to be included in the study.- Inclusion criteria may include factors such as age, sex, race, ethnicity, stage of disease, the subject’s past treatment history, E.T.C.
Example: “For participants to be included in this study, they have to be clients on 1st line-ART regimen for at least 3 years and are attending ART clinic at Kayunga Hospital during the time of data collection. They must also be of 18 years of age and above. Since 18 year of age is the consent age according to the Ugandan constitution”.
3.5 Definitions of Variables
- A measurable characteristic that assumes different values among the subjects
- It’s a value of interest to the researcher
- Basically variables can be;
- Dependant
- Independent
- Intervening
- Let the reader know what, (define), your dependent variable and independent variables of the study are.
For example; “the dependent variable of this study is the virological outcome (level of viral load). In this study the level of viral load means the amount (measure) of Plasma HIV-1 RNA. Viral load is measured in ml/copies. Viral load of >5000 copies/ml at 12 months of antiretroviral treatment will be taken as indication for virological failure (similar to WHO recommendation in resource- limited countries)”.
3.6 Research Instruments
- This refers to the tools you are going to use to answer your objectives
- They include;
- Questionnaires
- Interviews
- Checklists
- Standardized tests
Explain the instruments that will be used to collect data.
For, example: “The researcher will use a questionnaire which consists of both open and close ended questions written in simple language and will be filled by the researcher himself and his assistant by use of patient’s files and interview of clients. The questionnaire written by the researcher will be pretested to adjust for any ambiguity or errors and corrections will be made”.
Questionnaires: This mainly involves the use of pre-determined answers to gather information from participants
- It mainly has two forms
- Self administered
- Researcher administered
- Questions can be closed ended or open ended
Advantages and Disadvantages
| Self-Administered Questionnaires | Researcher-Administered Questionnaires | |
|---|---|---|
| Advantages | ||
| Convenience | Participants can complete at their own pace. | Researchers can clarify questions for better understanding. |
| Privacy | Respondents have privacy for sensitive questions. | Higher motivation can lead to improved response rates. |
| Time Flexibility | Participants can choose when to complete the survey. | Allows probing to ensure thorough and accurate responses. |
| Cost-Effective | No researcher presence reduces data collection costs. | Researchers can control the survey environment. |
| Large Sample Size | Suitable for reaching a larger, geographically spread sample. | Offers control over data quality and completeness. |
| Reduced Researcher Bias | Participants may provide candid responses. | Offers the ability to probe and clarify ambiguous answers. |
| Disadvantages | ||
| Non-Response Bias | Response rates might be lower, potentially biased. | Time-consuming due to researcher presence. |
| Misinterpretation | Participants might misunderstand questions. | Presence of a researcher can influence participant responses. |
| Incomplete Responses | Respondents may skip or provide incomplete answers. | Can be costly due to resources needed for administration. |
| Low Control | Researchers have limited control over survey environment. | Limited anonymity might affect the honesty of responses. |
| Limited Probing | Researchers cannot probe further for clarification. | Geographical constraints limit participant availability. |
Interviews: These are mainly used to get responses for qualitative data
- They could be used as;
- Interview guides.
- Focus Group discussion interviews- of 5 to 10 members.
Checklists: Also called observation forms.
- Researcher ticks responses on observation of what has been done or not.
- In many studies rating is done there after.
Standardized tests:
- These are tools used to score all populations across the board.
- For example when scoring IQ levels of children, cognitive tests.
3.7 Data Collection Procedures
- This involves the use of the selected tool/tools to gather information from the participants.
- It explains how the selected data tool will collect the information.
- These are selected depending on the design and approach selected.
- Here, you explain the whole procedure of data collection.
For Example: “A letter obtained from research committee will be taken to the management of Kayunga Hospital and to the ART clinic to allow researcher carry out data collection among HIV- infected clients on 1st
line ART regimens. One clinician will be identified from ART clinic and will be trained as a research assistant to help in filling in the questionnaires. A verbal and written consent will be obtained from respondents before data collection and an appreciation in form of thanks will be told to clients.”
3.7.1 Data Management
- This involves the cleaning of data to correct any missing errors.
- It involves pre cleaning before actual data entry to eliminate wrong data entry.
- Explain how data will be managed.
For, example: “After data collection, every questionnaire will be checked for completeness and any gaps will be filled immediately before the client leaves the clinic. The questionnaire will be kept under key and lock only accessible to the researcher and his assistant on request then it will directly be entered into SSPS soft ware package for social science version.”
3.7.2 Data Analysis
- After data has been cleaned, it then analyzed and interpreted to make meaningful statements.
- This is then followed by making interpretation of findings before the actual generalization of the research findings.
Explain how data will be analyzed.
For example, “Data will be entered directly into SPSS 17 for data analysis and will be analyzed starting with the demographic data and then the other objectives. The Analyzed data will then be presented in form of percentages and frequencies in tables, pie charts, and graphs”.
3.8 Ethical considerations
- This looks at the ethics of your research(Protection of Human Subjects)
- Informed consent
- Confidentiality
- Ethics committees
- Privacy
- Explain how you will meet the ethical guidelines of research.
For example: “Research proposal will be submitted to Research and Ethical Committee at Makerere University for approval. A letter from the Committee will be taken to Mulago Hospital management and ART clinic to seek permission to pre-test the Questionnaire. The same letter will be taken to Kayunga District hospital management and ART clinic where data collection will be done to seek permission to carry on data collection among HIV-infected clients on 1st —line ART regimens”.
3.9 Limitations of the Study
- These are anticipated challenges imposed by methods, period and location of research.
- The researcher may not have control over them and therefore the need to identify them so that possible solutions before beginning the study.
- They also help in predicting the necessary help need and the feasibility of the research.
- Explain the constraints you are like to meet and how you overcome them.
For example: “The researcher expects to encounter time constraints in the course of study, balancing the research study and other demanding work. The researcher will overcome this limitation by drawing up a time table that will be strictly followed”
3.10 Dissemination of Results
- Research findings must be shared to the relevant concerned bodies who might be interested in your findings.
- It can also be published as reports, journals, CMEs, posters in conferences etc.
- Dissemination helps other scholars know what has been done.
- List how and where you will communicate your results.
For example: “Information from the study wilt be compiled into a research report and four copies of research report will be made. A copy will be submitted to; Makerere University, Kayunga Hospital ART clinic, Research Supervisor and the Researcher.
References/Bibliography
- This includes all sources of cited, used and have been reffered too in he write up.
- It is a list of all authors whose work has been used in your proposal.
- This is written following the referencing guidelines of any institution.
- APA style is the preferred for our case.
Reference list:
This is an important part of the proposal. In the literature Review and in Background, you must have cited various authors. The page on References must show all the details about all the citations made in text.
Example;
References
- Byakwaga. Ff., murray,1., Petnumenos.K. (2E104). Prognosis of CPA in persons receiving ART. Aids Res Hum Retroviruses: 75(6):756-76
- CoIlia., Diedrichl. 6 JoAnna. (711U8). Unexpected low-level viremia among HIV-infected Ugandans adult with untreated active LB, i.acquir immune Defic synd.119:458
- Daar, E.. Mnudgil, T. 6 Meyer, R. (1991): Transient high levels of viremia in patients with primary human immunodeficiency virus type l infection. N Eng! J Med: 324(14):961-4
Appendices
- These extra things necessary for you to finalise your proposal
- They include;
- Budget
- Work plan
- Consent for patients
- Data collection tools
- Any other necessary document
Budget
| Budget | Example: |
|---|---|
| 1. Stationery | |
| – 10 reams of duplicating paper @shs 10,000 | 100,000 |
| – 3 boxes of pens @ 8,000 | 24,000 |
| Sub-total | 124,000 |
| 2. Travel | |
| – 5 return trips @ 10,000 | 50,000 |
| Sub-total | 50,000 |
| Total | 174,000 |
(Note: This example is for illustration purposes. Actual research budgets can vary significantly.)
Consent form:
Informed consent is the authorization granted with an awareness of the possible consequences, provided by the respondent to the researcher for their involvement in the study. (With a complete understanding of potential risks and benefits)
A consent form is the documentation that demonstrates the occurrence of the informed consent process.
Essential Components of the consent form:
- A clear and concise elucidation of the research’s purpose, incorporating the study’s title.
- An account of the procedures participants will undergo during the study, along with an indication of the time commitment for each element.
- Explanation of potential risks, side effects, or discomfort associated with the procedures.
- Detailing of potential benefits.
- Declaration that the participant’s engagement is voluntary, and they retain the right to withdraw without facing any repercussions.
- Assertion that the participant is permitted to raise inquiries regarding the study.
- Outline of the measures in place to safeguard participant confidentiality.
- Explanation of the data’s use after the study concludes.
- Confirmation that the participant shall receive a copy of the signed and dated consent form.
- Identification of the investigator(s) and contact information.
- A “statement of consent” along with the participant’s name and signature.
- Identification and signature of the person obtaining consent.
Example of a consent form
Consent Form
Introduction
Dear participant,
I am Tusing, a student pursuing a Diploma in Nuring at Nurses Revision school of Health Sciences. I am conducting a research study on the most effective first-line ART regimen among HIV-infected patients at Kayunga Hospital.
By participating in this study, you will contribute to preventing drug resistance and lowering mortality rates among HIV-infected patients at Kayunga Hospital. We do not foresee any risks to you during the course of this study.
Confidentiality: If you agree to participate in this study, the information obtained during the study will be kept confidential and will only be accessible to the researcher and the supervisors. Your name is also not needed on the questionnaire in order to participate in the study.
Voluntary Consent: You are free not to participate in the study and you have the right to refuse answering any question that you feel uncomfortable with. You are also free to withdraw from study at any time without fear of any consequences.
By signing below, you indicate your comprehension of the information provided about this study, and you willingly provide your consent to participate.
Name of respondent………………………….Signature of the respondent…………….Date…………….
Name of researcher…………………………Signature of the
researcher………………Date…………
Workplan
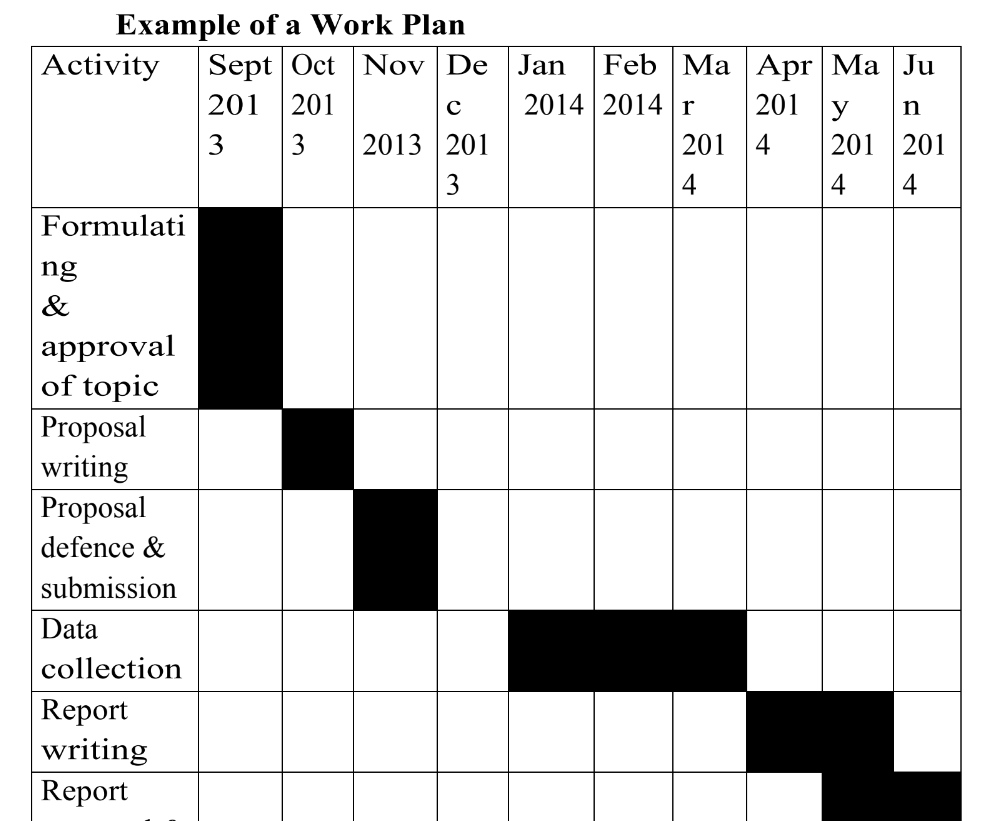


Wow, thanks so much for knowledge,I have really attained alot
Thanks for the update and good heart u have and may the Lord bless u full fill your hearts desires