- Infant respiratory distress syndrome (IRDS), also called neonatal respiratory distress syndrome, (previously called hyaline membrane disease (HMD), is a syndrome in premature infants caused by developmental insufficiency of pulmonary surfactant production and structural immaturity in the lungs.
- Respiratory distress syndrome (RDS) occurs in babies born early (premature) whose lungs are not fully developed. The earlier the infant is born, the more likely it is for them to have respiratory distress syndrome RDS and need extra oxygen and help breathing.
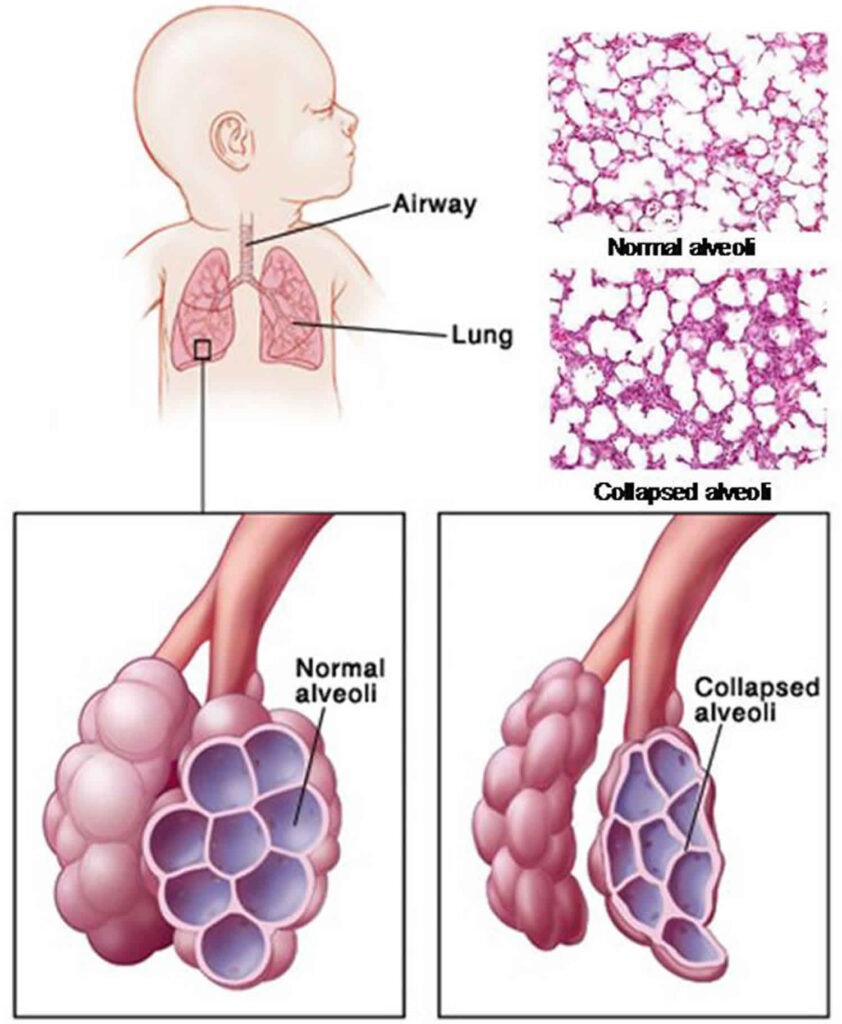
- RDS is caused by the baby not having enough surfactant in the lungs. Surfactant is a liquid made in the lungs at about 26 weeks of pregnancy. As the fetus grows, the lungs make more surfactant.
- Surfactant is a liquid that coats the inside of the lungs. It helps keep them open so that infants can breathe in air once they are born.
Table of Contents
ToggleCauses of Respiratory Distress Syndrome
- Lack or insufficient surfactant
- It can also be a consequence of neonatal infection.
- It can also result from a genetic problem with the production of surfactant associated proteins.
Risk Factors
- Premature birth (before 37 weeks)
- A sibling with respiratory distress syndrome
- Multiple pregnancy (twins, triplets)
- Impaired blood flow to the baby during delivery
- Delivery by cesarean
- Maternal diabetes
- infection
- Induction of labor before the baby is full-term
- Multiple pregnancy (twins or more)
- Cold stress. Baby with trouble of maintaining body temperature
- Patent ductus arteriosus
- Rapid labor
- Prematurity
Pathophysiology
The lungs of infants with respiratory distress syndrome are developmentally deficient in a material called surfactant, which helps prevent collapse of the terminal air-spaces throughout the normal cycle of inhalation and exhalation.
This deficiency of surfactant is related to an inhibition from the insulin that is produced in the newborn especially in diabetic mothers. Deficient surfactant production causes un equal inflation of alveoli on inspiration and collapse of alveoli on end of expiration.
In this case their lungs inflate and therefore exert a great deal of effort to re-expand the alveoli with each breath with increasing exhaustion, they will be able to open the alveoli.
Inability to maintain lung expansion produces a wide spread of atelectasis. Progressive atelectasis with absence of alveolar stability will lead to increased pulmonary vascular resistance where as in normal cases it is supposed to decrease. Consequently there will be hypertension to the lung tissue a (pulmonary hypertension)decrease in effective pulmonary blood flow.

Phases of ARDS (Pathogenesis)
ARDS has three phases—exudative, proliferative, and fibrotic.
1. Exudative Phase
In this phase, alveolar capillary endothelial cells and type I pneumocytes (alveolar epithelial cells) are injured, and tight alveolar barrier is damaged giving away the entry to fluid and macromolecules. The protein rich edema fluid accumulates in the interstitial and alveolar spaces. Pro-inflammatory cytokines are increased in this acute phase, leading to the recruitment of leukocytes (especially neutrophils) into the pulmonary space and alveoli. There is plasma proteins aggregation in air spaces with cellular debris and dysfunctional pulmonary surfactant to form hyaline membrane whorls of which Alveolar edema predominantly leads to lung diminished aeration. Collapse of large sections of dependent lung can contribute to decreased lung compliance. It causes intrapulmonary shunting and hypoxemia develop and the work of breathing increases, leading to dyspnea.
The exudative phase encompasses the first 7 days of illness after exposure to a precipitating ARDS risk factor. Tachypnea and increased work of breathing result frequently in respiratory fatigue and ultimately in respiratory failure.
2. Proliferative Phase
This phase of ARDS usually lasts from day 7 to day 21. Most patients recover rapidly and are liberated from mechanical ventilation during this phase. Despite this improvement, many patients still experience dyspnea, tachypnea, and hypoxemia. Histologically, the first signs of resolution are often evident in this phase, with the initiation of lung repair, the organization of alveolar exudates, and a shift from neutrophil- to lymphocyte- pulmonary infiltrates.
As part of the reparative process, type II pneumocytes proliferate along alveolar basement membranes. These specialized epithelial cells synthesize new pulmonary surfactant and differentiate into type I pneumocytes.
3. Fibrotic Phase
Most patients with ARDS recover lung function within 3–4 weeks, very few progresses into fibrotic phase that may require long-term support on mechanical ventilators and/or supplemental oxygen. There is extensive alveolar-duct and interstitial fibrosis. Marked disruption of acinar architecture leads to emphysema-like changes, with large bullae.
Intimal fibroproliferation in the pulmonary microcirculation causes progressive vascular occlusion and pulmonary hypertension. The physiologic consequences include an increased risk of pneumothorax, reductions in lung compliance, and increased pulmonary dead space.
Signs and Symptoms
- Infant respiratory distress syndrome begins shortly after birth
- Fast breathing
- Fast heart rate
- Chest wall retractions (recession)
- Expiratory grunting
- Nasal flaring
- Cyanosis
- Ventilatory failure (rising carbon dioxide concentrations in the blood) as condition progresses
- Prolonged cessations of breathing (“apnea”).
- Reduced urine output
Diagnosis/Investigation
- Signs and symptoms
- Chest x-ray
- Pulse Oximetry
- Echocardiography
- CT scans
- Arterial blood gas (ABG) test to assess the level of oxygen, CO2, and acids in blood
Differential Diagnosis
- Acute Anemia
- Aspiration Syndromes
- Pediatric Gastroesophageal Reflux
- Pediatric Hypoglycemia
- Pediatric Pneumonia
- Pediatric Polycythemia
- Pneumomediastinum
- Pneumothorax
- Transient Tachypnea of the Newborn
Management / Treatment
- Delivery and resuscitation: A neonatologist experienced in the resuscitation and care of premature infants should attend the deliveries of fetuses born at less than 28 weeks’ gestation.
- Keep the child warm
- Oxygen is given with a small amount of continuous positive airway pressure
- I.V. fluids (N/S, D5%; (Neonatalyte i.e. D50%= 70mls, D5% = 310 & R/L=120ML) are administered to stabilize the blood sugar, blood salts, and blood pressure.
- In severe cases an endotracheal tube is inserted into the trachea and intermittent breaths are given by a mechanical device.
- A preparation of surfactant (e.g. survanta or beraksurf), is given through the breathing tube into the lungs.
- Administer a glucocorticoid e.g. dexamethasone (0.15mg /kg/dose; max dose 4mg)
- Give an antibiotic to prevent secondary bacterial infection
- Respiratory monitoring, pulse rate, Bp, temperature, ECG monitoring.
- Monitor conscious level
- Reassure the mother
- NG tube feeding
- Vitamin k 0.5-1mgm I.M due to risk of intraventricular hemorrhage.
Prevention
- Giving the mother glucocorticoids speeds the production of surfactant. Glucocorticoid treatment is recommended for women at risk for preterm delivery prior to 34 weeks of gestation (dose 12-40mg)
- Early antenal care
- Eat healthy diet rich in vitamins
- Avoid smoking and alcohol during pregnancy
Complications
- Metabolic disorders (acidosis, low blood sugar)
- Patent ductus arteriosus
- Low blood pressure
- Chronic lung changes
- Bleeding in the brain.
CASE SCENARIO
- A 1-day-old boy is brought to the intensive care unit from the nursery due to increased work of breathing. The patient was born at 31 weeks to a mother with a history of multiple preterm deliveries, polysubstance abuse and HIV. His temperature is 38°C (100.4°F), pulse is 215/min, respirations are 76/min, blood pressure is 60/41 mmHg, and oxygen saturation is 85% on room air. Physical exam shows tachypnea, nasal flaring, and subcostal retractions. Administration of supplemental oxygen and positive pressure ventilation improve the patient’s oxygen saturation to 95%. Blood glucose is 95 mg/dL. Chest x-ray and laboratory results are shown below:
| Laboratory value | Result |
| Blood Gases, Serum | |
| pH | 7.23 |
| PCO2 | 55 mmHg |
| PO2 | 30 mmHg |
Which of the following best describes the etiology of this infant’s disease process?
2. Mike, a 55-year-old man, presents with shortness of breath, high fever, and cough. A chest x-ray was ordered and it showed a right lower lobe infiltrate, which is suggestive of pneumonia. He was then started on IV antibiotics but the following day Mike became hypoxic and hypotensive. Because his hypotension didn’t improve despite intubation, IV fluids, and vasopressors, he is diagnosed with septic shock. Next, a repeat x-ray detected newly-developed bilateral alveolar opacities, heart echography ruled out heart failure, and arterial blood gas analysis revealed a PF ratio of 109 milligrams Mercury.
3. Dona, an infant delivered by cesarean section at 36 weeks’ gestational age, with an Apgar score of 9 at birth. A few hours after delivery, she develops tachypnea, chest wall retractions with nasal flaring, and tachycardia. Aside from increased work of breathing, her physical examination findings are normal. A chest x-ray was ordered and it showed diffuse reticulogranular ground glass appearance with air bronchograms.
Detailed Review.
All the above scenerio’s point to respiratory distress syndrome
But first, a bit of physiology.
Normally, when you breathe in, the air reaches the alveoli, which are made up of two types of pneumocytes.
First, type I pneumocytes are thin, and have a large surface area that that facilitate gas exchange.
More important for the exams are the type II pneumocytes, which are smaller, thicker and have the ability to proliferate in response to lung injury.
They are in charge of making a fluid called surfactant which contains various phospholipids. This lets it act like droplets of oil that coats the inside of the alveoli, decreasing surface tension, so if it’s missing, the alveoli will collapse.
These cells also act like stem cells, meaning they can give rise to type I cells and type II pneumocytes.
Ok, so acute respiratory distress syndrome, or ARDS, is characterized by rapid onset of widespread inflammation in the lungs which can lead to respiratory failure.
ARDS is not a primary disease, as it is usually triggered by conditions like sepsis, aspiration, trauma, and pancreatitis.
Now ARDS starts when these conditions cause alveolar damage, and a high yield fact is that the injury triggers the pneumocytes to secrete inflammatory cytokines like TNF-alpha and interleukin 1.
This subsequently leads to neutrophil recruitment, and they will release toxic mediators, like reactive oxygen species and proteases, which will damage the lungs even more.
You’ll need to know that the main site of injury is the alveolar-capillary membrane, which becomes more permeable, causing fluid to move into the alveoli resulting in pulmonary edema. This fluid can impair gas exchange, leading to hypoxemia.
Furthermore, the edema can also wash away the surfactant coating the alveoli to the point where it can’t reduce surface tension anymore, and as a result, the alveoli collapse.
And finally, dead cells and protein-rich fluid start to pile up in the alveolar space and, over time, it forms these waxy hyaline membranes which look like a layer of glassy material.
Individuals with ARDS present with serious symptoms and signs that require urgent investigation. The inflammation process and impaired gas exchange lead to fever, shortness of breath, tachypnea, chest pain, hypotension, hypoxia, and cyanosis. More often than not, ARDS will lead to shock due to hypotension.
The excess fluid in the lungs can cause a crackling sound called rales during auscultation, which is the sound of collapsed alveoli popping open with inspiration.
Keep in mind additional symptoms might provide clues to the underlying cause.
For example, epigastric abdominal pain radiating to the back along with a history of gallstones indicate acute pancreatitis. Diagnosis of ARDS is typically made when the individual presents all of the next four criteria, which you should definitely remember for your exams. First, the symptoms have to be “acute” meaning an onset of one week or less.
Second, and particularly high yield, a chest X-Ray or CT scan shows opacities or “white out” in both lungs, which is due to pulmonary edema.
The third is what’s called the PF ratio. It’s the partial pressure of oxygen in the arterial blood divided by the percent of oxygen in the inspired air, also called the fraction of inspired oxygen.
In ARDS, gas exchange is defective so the PF ratio is below 300 mmHg, and the lower this ratio gets, the more severe the condition.
Fourth, the respiratory distress must not be due to cardiac causes, like heart failure.
Often this is assessed by using an echocardiogram to look for evidence of heart failure, like an ejection fraction below 55% in systolic heart failure, and abnormal relaxation of the myocardium in diastolic heart failure.
Another clue is the pulmonary capillary wedge pressure, which is measured by inserting a catheter into a small pulmonary arterial branch.
In heart failure, this is elevated because more blood remains in the left side of the heart and it prevents pulmonary venous return.
The blood backs up into the pulmonary vessels, and the increase in pressure pushes fluid into the interstitial space of the lungs, resulting in edema.
In ARDS, the pressure is normal since the edema is caused by leaky capillaries instead of increased pressure.
Treatment of ARDS ultimately comes down to treating the condition that triggered it. However, the most important initial step is supportive care, like supplemental oxygen or mechanical ventilation.
A high yield fact to remember is that it’s vital to maintain positive end-expiratory pressure, which is where the pressure in the lungs is kept slightly above atmospheric pressure, even after exhalation, because this prevents the alveoli from collapsing. It’s also good to have low tidal volumes to prevent over-inflation of the damaged alveoli. Another important thing to watch out for is positive pressure ventilation can cause compression of pulmonary vessels which leads to pulmonary hypertension decreased pulmonary venous return.
This will reduce cardiac output and hypotension might worsen.

Pingback: Pulmonary Hemorrhage - Nurses Revision
I enjoyed reading the notes…
They are so good,very easy to read and understand…
Thanks so much for making our reading so easy…
Simple notes easy to understand, thanks for the good work,, God bless
Thanks for helping us, we’re really acquiring some knowledge
Thanks for the simplified notes, easy to understand something.
So fablastic notes, could we have more of the nursing interventios in the management of RDS