Fractures
Fractures
- Fractures are complete or incomplete disruption in the continuity of the bone.
- A fracture is a break in the continuity of a bone tissue, when subjected to excessive abnormal force or
- A fracture is a break in the bone that occurs when more force is applied to the bone than the bone can withstand.
- Fractures are also known as broken bones.
Common Childhood Fractures.
- Arm bones are fractured more often than other bones.
- Collarbone or shoulder fractures
- Elbow fractures
- forearm, wrist, or hand fracture
- Leg, foot, or ankle fracture.
Causes of Fractures
- Direct Force: in which the fracture occurs at the point of contact.
- Torsion: in which the fracture occurs at the point opposite the location of the force, e.g. twisting of the foot may lead to break of bones of the leg.
- Violent Contractions: e.g. forcibly throwing an object produces powerful muscle contractions which can fracture the humerus. Also in strong contractions in tetanus.
- Disease Processes: cause weakening of the bone structure; osteoporosis, malnutrition, bone tumors
Risks for fractures
- Sporting accidents
- Falls from heights
- Bike and car accidents
- Poor nutrition; a diet low in calcium
Associated events following fractures.
When a bone is broken adjacent structures are also affected, resulting in;
- Soft tissue edema
- Joint dislocation
- Ruptured tendons
- Severed nerves
- Damaged blood vessels
- Hemorrhage into the muscles & joints
Classification of Fractures
Fractures can be grouped into 4 categories; i.e
- Communication with environment.
- By Anatomical Site
- By Pattern
- Miscellaneous
COMMUNICATION WITH ENVIRONMENT
- Open/compound fracture: fractured part is exposed to the external environment. These are fractures where the bone is exposed through the skin or mucous membrane.
- Closed/simple fracture: fracture with the overlying skin intact. These are types of fractures that do not penetrate the skin.
- Complete fracture/Incomplete: Foe complete, the fracture line runs entirely through the bone substance with the periosteum disrupted on both sides of the bone and 2 fragments are present on either side of the fracture line. In incomplete, the fracture doesn’t entirely destroy the continuity of the bone.
- Avulsion: A fracture occurring from pulling effects of ligaments and tendons.
- Potts Fracture : Type of fracture that occurs at the ankle joint.
- Colles fracture(distal radius fracture): a fracture that occurs at the wrist joint.
- Transverse : where the fracture in the bone is broken perpendicular to its length i.e. right angle to the bone.
- Oblique: where the fracture extends in an oblique direction.
- Spiral: fracture in which the born has been twisted apart.
- Green stick fracture: common in children and bone commonly bends or in severe cases, fracture line extends only half way across the bone substance. One side of the bone is broken, causing the other side to bend. A greenstick fracture resembles a broken tree branch. The branch cracks on one side but remains partially intact on the other.
- Depressed fracture: a fracture having its edges driven below the surrounding bone surfaces, e.g. fractured skull.
- Comminuted fracture: bone fragments are crushed or broken into small pieces. Occurs after high impact trauma eg a vehical road crash.
- Displaced/overriding fracture: the bone fragments are separated away from the fracture line and bone ends are overlapping each other.
- Impacted: where a bone fragment is moved into another.
- Complicated fracture: that which is associated with many structures destroyed such as nerves, blood vessels, joints, muscles
- Stress fracture: occurs on a normal or abnormal bone from constant exposure to stress e.g. running a long distance, jumping a rope
- Pathological fracture: a fracture caused by a disease e.g bone cysts, paget’s disease.
Signs and symptoms of fractures
- Pain at the site of injury : which is continuous and increases in severity until the bone fragments are immobilized.
- Local tenderness.
- Deformity : displacements, rotation of fragments in the fracture of the limb causes deformity(either palpable/visible). Is detectable when limb is compared with the uninjured extremity.
- Soft tissue swelling
- Loss of function : after the fracture, the extremity cannot function properly because normal function of muscles depends on the integrity of the bones to which they are attached.
- Bruising
- Involuntary muscle spasms.
- Intense pain e.g. in the rib cage when a patient takes a deep breath or coughs.
- Abnormal mobility
- Involuntary muscle spasms
- Crepitus – when the extremity is examined with the hands, a grating sound or sensation is heard or felt when broken ends rub on each other and is called crepitus.
- Bone may be visibly seen protruding through the skin.
- Impaired sensation/numbness may occur if there is nervous damage
- Shock results from blood loss
- Swelling and discoloration: localized swelling and discoloration of the skin (Ecchymosis) occurs after a fracture, as a result of trauma and bleeding into the tissues.
Assessment and Diagnostic Findings
To determine the presence of fracture, the following diagnostic tools are used.
- History taking.
- Physical Examinations.
- X-ray examinations: Determines location and extent of fracture.
- Bone scans, computed tomography (CT)/magnetic resonance imaging (MRI) scans: Visualizes fractures, bleeding, and soft-tissue damage.
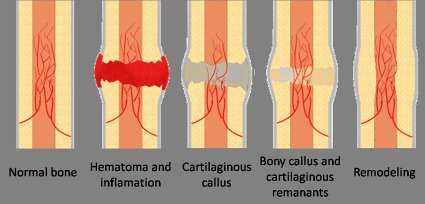
Process of Fracture Healing
This process varies according to the type of bone involved type of fracture and the amount of movement at the fracture site. In the absence of rigid fixation in tubular bones, healing proceeds in five stages as follows;
1.Tissue destruction & haematoma formation:
After tissue destruction, torn blood vessels result to hematoma formation (which is a collection of clotted blood between the ends of the bones and in surrounding soft tissues. Fibrin, red blood cells, debris and inflammatory exudates come together and form a fibrin clot.
2.Inflammation & cellular proliferation:
This follows development of acute inflammation and accumulation of inflammatory exudate containing macrophages that phagocytose the hematoma and small fragments of bone without blood supply and this takes about 5 days. Fibroblasts migrate to the site, granulation tissue and new capillaries develop.
3. Stage of Callus formation (Soft Callus)
New bone forms as large number of osteoblasts secrete spongy bone, which unites the broken ends, osteoclasts begin mopping up the dead bone. The new deposits of bone and cartilage are called callus. As the immature bone (soft callus) becomes more densely mineralized, movement of the site progressively decreases.
4.Stage of consolidation(Hard callus)
Over the next few days, callus matures and the cartilage is gradually replaced with new bone.
5.Stage of remodelling
This is the reshaping of the callus by a continuous process of resorption and laydown. Internal callus is hollowed out into marrow cavity, while external callus is slowly removed. Reshaping of the bone continues and gradually the medullary canal is reopened through the callus, and callus tissue is completely replaced with mature compact bone. Often the bone is thicker and stronger at the repair site than originally and a second fracture is more likely to occur at a different site.
Healing of Fractures
Factors necessary for bone healing
- Haematoma formation.
- Contact of bone end and no interposition of other tissues
- Continued immobilization until calus is able to withstand stress.
- Good supply of blood
- Enough rest of the fractured site
- Good nutrition- calcium, proteins
Factors influencing bone healing.
(a). Systemic factors
- -Age ( healing is almost twice as fast in children as in adults )
- -Activity level.( immobilization)
- -Nutritional status.
- -Hormonal factors (GH, corticosteroids )
- -Diseases e.g. DM, anaemia, neuropathies
- -Vitamin deficiencies e.g. A C D K
- -Drugs e.g. anti coagulants, anti inflammatory.
(b). Local factors.
- -type of bone( cancellous heals faster than cortical bone)
- -type of fracture. Spiral better than transverse.
- -blood supply ( poor circulation-poor healing.)
- -reduction- faster when there’s perfect reduction.
- -infection
- -soft tissue interposition
- -mobilization. Early vs late mobilization.
Management
Aims of management.
- To regain and maintain the normal alignment of the injured part.
- To regain normal function of the injured part.
- To achieve the above objectives for the patient in the shortest time possible
Basic principles of managing fractures
The principles of fracture management are:
- Reduction
- Immobilization
- Rehabilitation
(1) Reduction: Reduction is the process of restoring the bone ends (and any fractured fragments) into their normal anatomical positions. This is accomplished by open or closed manipulation of the affected area, referred to as open reduction and closed reduction.
- Closed reduction is accomplished by bringing the bone ends into alignment by manipulation and manual traction. X-rays are taken to determine the position of the bones. A cast is normally applied to immobilize the extremity and maintain the reduction.
- In open reduction, a surgical opening is made, allowing the bones to be reduced manually under direct visualization. Frequently, internal fixation devices will be used to maintain the bone fragments in reduction.
(2) Immobilization: Immobilization is necessary to maintain fracture reduction until healing occurs. Immobilization may be accomplished by external or internal fixation.
- Methods of external fixation include casts, splints, and continuous traction.
- Internal fixation devices include pins, wires, screws, rods, nails, and plates.
(3) Rehabilitation: Rehabilitation is the regaining of strength and normal function in the affected area. Specific rehabilitation for each patient will be based upon the type of fracture and the methods of reduction and immobilization used.
First aid management
This can save the client’s life so it is crucial. A B C D of life criteria is used as follows;
Ensure that the patient is breathing then do the following:
- Airway should be clear
- Breathing should be maintained.
- Check pulse and control bleeding.
- Deformity should immobilized, Immobilize the limb to avoid more harm and pain by using splints.
Emergency Management of Fractures
- Immediately after injury, whenever a fracture is suspected, it is important to immobilize the body part before the patient is moved.
- If an injured patient must be removed from a vehicle before splints can be applied, the extremity is supported above and below the fracture site to prevent rotation as well as angular motion.
- Adequate splinting, including joints adjacent to the fracture, is essential.
- Movement of fracture fragments causes additional pain, soft tissue damage, and bleeding.
- Temporary, well-padded splints, firmly bandaged over clothing, serve to immobilize the fracture.
- Immobilization of the long bones of the lower extremities may be accomplished by bandaging the legs together, with the unaffected extremity serving as a splint for the injured one.
- In an upper extremity injury, the arm may be bandaged to the chest, or an injured forearm may be placed in a sling.
- The neurovascular status distal to the injury should be assessed to determine adequacy of peripheral tissue perfusion and nerve function.
- With an open fracture, the wound is covered with a clean (sterile) dressing to prevent contamination of deeper tissues.
- No attempt is made to reduce the fracture, even if one of the bone fragments is protruding through the wound.
- Splints are applied for immobilization.
- In the emergency department, the patient is evaluated completely.
- The clothes are gently removed, first from the uninjured side of the body and then from the injured side.
- The patient’s clothing may be cut away. The fractured extremity is moved as little as possible to avoid more damage
Management in hospital
- Care depends on class/type of fracture
- Immobilization/ reduction and rehabilitation is done.
- Pain relief
- Anti biotics
- Supportive treatment eg feso4,FA ,multivitamin
- Bone x- ray
- Fluid resuscitation
- Infection prevention
- Nutrition – calcium
- Exercises/ physiotherapy
- Nursing care
Nursing Care
- Encourage patients with closed (simple) fractures to return to their usual activities as rapidly as possible.
- Teach patients how to control swelling and pain associated with the fracture and with soft tissue trauma and encourages them to be active within the limits of the fracture immobilization.
- It is important to teach exercises to maintain the health of unaffected muscles and to increase the strength of muscles needed for transferring and for using assistive devices (eg, crutches, walker, special utensils).
- Teach patients to use assistive devices safely. Plans are made to help patients modify their home environment as needed and to secure personal assistance if necessary.
- Patient teaching include self-care, medication information, monitoring for potential complications and the need for continuing health care supervision.
- Flracture healing and restoration of full strength and mobility may take months
Managing Fractures at Specific Sites
Maximum functional recovery is the goal of management.
Clavicle
- Fracture of the clavicle (collar bone) is a common injury that results from a fall or a direct blow to the shoulder.
- Monitor the circulation and nerve function of the affected arm and compare with the unaffected arm to determine variations, which may indicate disturbances in neurovascular status.
- Caution the patient not to elevate the arm above shoulder level until the fracture has healed (about 6 weeks).
- Encourage the patient to exercise the elbow, wrist, and fingers as soon as possible and, when prescribed, to perform shoulder exercises.
- Tell the patient that vigorous activity is limited for 3 months.
Humeral Neck
- With humeral neck fractures (seen most frequently in older women after a fall on an outstretched arm), perform neurovascular assessment of the involved extremity to evaluate the extent of injury and possible involvement of the nerves and blood vessels of the arm.
- Teach the patient to support the arm and immobilize it by a sling and swathe that secure the supported arm to the trunk.
- Begin pendulum exercises as soon as tolerated by the patient. Instruct the patient to avoid vigorous activity for an additional 10 to 14 weeks.
- Inform the patient that residual stiffness, aching, and some limitation of range of motion may persist for 6 or more months.
- When a humeral neck fracture is displaced with required fixation, exercises are started only after a prescribed period of immobilization.
Humeral shaft fractures
- The nerves and brachial blood vessels may be injured, so neurovascular assessment is essential to monitor the status of the nerve or blood vessels.
- Use well-padded splints to initially immobilize the upper arm and to support the arm in 90 degrees of flexion at the elbow, use a sling or collar and cuff to support the forearm, and use external fixators to treat open fractures of the humeral shaft.
- Functional bracing may also be used for these fractures.
- Teach patient to perform pendulum shoulder exercises and isometric exercises as prescribed.
Elbow
- Elbow fractures (distal humerus) may result in injury to the median, radial, or ulnar nerves.
- Evaluate the patient for paresthesia and signs of compromised circulation in the forearm and hand.
- Monitor closely for Volkmann’s ischemic contracture (an acute compartment syndrome) as well as for hemarthrosis (blood in the joint).
- Reinforce information regarding reduction and fixation of the fracture and planned active motion when swelling has subsided and healing has begun.
- Explain care if the arm is immobilized in a cast or posterior splint with a sling. Encourage active finger exercises.
- Teach and encourage patient to do gentle range of motion exercise of the injured joint about 1 week after internal fixation.
Radial head fractures
- They are usually produced by a fall on the outstretched hand with the elbow extended.
- Instruct patient in use of a splint for immobilization.
- If the fracture is displaced, reinforce the need for postoperative immobilization of the arm in a posterior plaster splint and sling.
- Encourage the patient to carry out a program of active motion of the elbow and forearm when prescribed
Wrist
- Wrist fractures (distal radius [Colles’ fracture]) usually result from a fall on an open, dorsiflexed hand.
- They are frequently seen in elderly women with osteoporotic bones and weak soft tissues that do not dissipate the energy of a fall.
- Reinforce care of the cast, or with more severe fractures with wire insertion, teach incision care.
- Instruct patient to keep the wrist and forearm elevated for 48 hours after reduction.
- Begin active motion of the fingers and shoulder promptly by teaching patient to do the following exercises to reduce swelling and prevent stiffness:
- Hold the hand at the level of the heart. Move the fingers from full extension to flexion. Hold and release. Repeat at least 10 times every hour when awake.
- Use the hand in functional activities.
- Actively exercise the shoulder and elbow, including complete range-of-motion exercises of both joints
- Assess the sensory function of the median nerve by pricking the distal aspect of the index finger, and assess the motor function by testing patient’s ability to touch the thumb tot he little finger.
- If diminished circulation and nerve function is noted, treat promptly.
Hand and Fingers
- Hand trauma often requires extensive reconstructive surgery.
- The objective of treatment is always to regain maximum function of the hand.
- With a non displaced fracture, the finger is splinted for 3 to 4 weeks to relieve pain and protect the fingertip from further trauma, but displaced fractures and open fractures may require open reduction with internal fixation, using wires or pins.
- Encourage functional use of the uninvolved portions of the hand
- Evaluate the neurovascular status of the injured hand.
- Teach the patient to control swelling by elevating the hand.
Pelvis
- Pelvic fractures may be caused by falls, motor vehicle crashes, or crush injuries. At least two thirds of these patients have significant and multiple injuries.
- Monitor for symptoms, including ecchymosis; tenderness over the symphysis pubis, anterior iliac spines, iliac crest, sacrum, or coccyx; local edema; numbness or tingling of the pubis, genitals, and proximal thighs; and inability to bear weight without discomfort.
- Complete a neurovascular assessment of the lower extremities to detect injury to pelvic blood vessels and nerves.
- As pain resolves, instruct patient to resume activity gradually, using assistive mobility devices for protected weight bearing. Patients with unstable pelvic fractures may be treated with external fixation or open reduction and internal fixation (ORIF).
- Promote hemodynamic stability and comfort, and encourage early mobilization.
- Examine urine for blood to assess for urinary tract injury. In male patients, do not insert a catheter until the status of the urethra is known.
- Monitor for diffuse and intense abdominal pain, hyperactive or absent bowel sounds, and abdominal rigidity and resonance (free air) or dullness to percussion (blood), which suggest injury to the intestines or abdominal bleeding.
- Monitor for hemorrhage and shock, two of the most serious consequences that may occur. Palpate both lower extremities for absence of peripheral pulses, which may indicate a torn iliac artery or one of its branches.
- Assess for injuries to the bladder, rectum, intestines, other abdominal organs, and pelvic vessels and nerves.
- If patient has a stable pelvic fracture, maintain patient on bed rest for a few days and provide symptom management until the pain and discomfort are controlled.
- Provide fluids, dietary fiber, ankle and leg exercises, antiembolism stockings to aid venous return, logrolling, deep breathing, and skin care to reduce the risk for complications and to increase comfort.
- Monitor bowel sounds. If patient has a fracture of the coccyx and experiences pain on sitting and with defecation, assist with sitz baths as prescribed to relieve pain, and administer stool softeners to prevent the need to strain on defecation.
Femur and Hip
- Femoral shaft fractures are most often seen in young adults involved in a motor vehicle crash or a fall from a high place.
- Frequently, these patients have associated multiple trauma and develop shock from a loss of 2 to 3 units of blood.
- Assess neurovascular status of the extremity, especially circulatory perfusion of the lower leg and foot (popliteal, posterior tibial, and pedal pulses and toe capillary refill time as well as Doppler ultrasound monitoring).
- Note signs of dislocation of the hip and knee, and knee effusion, which may suggest ligament damage and possible instability of the knee joint
- Apply and maintain skeletal traction or splint to achieve muscle relaxation and alignment of the fracture fragments before ORIF procedures, and later a cast brace.
- Assist patient in minimal partial weight bearing when indicated and progress to full weight bearing as tolerated.
- Reinforce that the cast brace is worn for 12 to 14 weeks
- Instruct in and encourage patient to perform exercises of lower leg, foot, and toes on a regular basis.
- Assist patient in performing active and passive knee exercises as soon as possible, depending on the management approach and the stability of the fracture and knee ligaments.
Tibia and Fibula
- Tibia and fibula fractures (most common fractures below the knee) tend to result from a direct blow, falls with the foot in a flexed position, or a violent twisting motion.
- Provide instruction on care of the long leg walking cast or patellar-tendon-bearing cast.
- Instruct patient in and assist with partial weight bearing,
- usually in 7 to 10 days.
- Instruct patient on care of a short leg cast or brace (in 3 to 4 weeks), which allows for knee motion.
- Instruct patient in care of skeletal traction, if applicable.
- Encourage patient to perform hip, foot, and knee exercises within the limits of the immobilizing device.
- Instruct patient to begin weight bearing when prescribed (usually in about 4 to 8 weeks).
- Instruct patient to elevate extremity to control edema.
- Perform continuous neurovascular evaluation.
Rib
- Rib fractures occur frequently in adults and usually result in no impairment of function but produce painful respirations.
- Assist patient to cough and take deep breaths by splinting the chest with hands or pillow during cough.
- Reassure patient that pain associated with rib fracture diminishes significantly in 3 or 4 days, and the fracture heals within 6 weeks.
- Monitor for complications, which may include atelectasis, pneumonia, a flail chest, pneumothorax, and hemothorax.
COMPLICATIONS OF FRACTURES
Early complications include;
- Shock,
- Fat embolism,
- Compartment syndrome, and
- Venous thromboembolism (deep vein thrombosis [DVT],
- Pulmonary embolism [PE]).
Delayed Complications;
- Delayed union,
- Malunion,
- Nonunion,
- Avascular necrosis (AVN) of bone, reaction to internal fixation devices
- Complex regional pain syndrome (CRPS, formerly called reflex sympathetic dystrophy (RSD), is chronic condition of severe burning pain affecting one of the extremities
- Heterotopic ossification- is the presence of bone in the soft tissue where bone normally does not exist
MANIFESTATION OF COMPLICATIONS
Fat embolism syndrome:
- Occurs with blockage of the small blood vessels that supply the brain, lungs, kidneys, and other organs.
- Sudden onset, usually occurring within 12 to 48 hour but may occur up to 10 days after injury),
- Signs & symptoms: hypoxia, tachypnea, tachycardia, and pyrexia; dyspnea, crackles, wheezes, precordial chest pain, cough, large amounts of thick white sputum.
Compartment syndrome
- Acute compartment syndrome may produce deep, throbbing, unrelenting pain not controlled by opioids
- It can be due to a tight cast or constrictive dressing or an increase in muscle compartment contents because of edema or hemorrhage.
- Signs & symptoms include; Cyanotic (blue-tinged) nail beds and pale or dusky and cold fingers or toes are present; nail bed capillary refill times are prolonged (greater than 3 seconds); pulse may be diminished or absent; and motor weakness, paralysis, and paresthesia may occur.
DIC- disseminated intravascular coagulation is evidenced by;
- Unexpected bleeding after surgery and
- Bleeding from the mucous membranes,
- Venipuncture sites, and
- Gastrointestinal and urinary tracts.
Infection. symptoms include.
- Tenderness on examination
- Pain (patient history)
- Redness,
- Swelling,
- Local warmth,
- Elevated temperature, and
- Purulent drainage.
Nonunion is manifested by
- Persistent discomfort and abnormal movement at the fracture site.
Some risk factors include;
- Infection at the fracture site
- Interposition of tissue between the bone ends
- Inadequate immobilization or manipulation that disrupts callus formation,
MANAGEMENT OF COMPLICATIONS
Treatment of shock :
- Consists of stabilizing the fracture to prevent further hemorrhage
- Restoring blood volume and circulation
- Relieving the patient’s pain
- Providing proper immobilization and
- Protecting the patient from further injury and other complications.
Prevention and management of fat embolism:
- Include immediate immobilization of fractures
- Adequate support for fractured bones during turning and positioning
- Maintenance of fluid and electrolyte balance
- Prompt initiation of respiratory support with prevention of respiratory and metabolic acidosis
- Corticosteroids as well as vasopressor medications may be given.
Compartment syndrome:
- Is managed by controlling swelling by elevating the extremity to heart level or by releasing restrictive devices (dressings or cast).
- A fasciotomy (surgical decompression with excision of the fascia) may be needed to relieve the constrictive muscle fascia.
- The wound remains open and covered with moist sterile saline dressings for 3 to 5 days.
- The limb is splinted and elevated.
- Prescribed passive range-of-motion exercises may be performed every 4 to 6 hours.
Nonunion (failure of the ends of a fractured bone to unite)
- Is treated with internal fixation
- Bone grafting
- Electrical bone stimulation, or a combination of these.
Related Question
Josephine a thirty year old female patient has been involved in a road traffic accident and sustained a compound fracture.
- Outline ten signs and symptoms of fracture.
- Discuss the negative factors that can influence healing of a bone.
- Describe the healing of a bone.
- Mention ten complications of fractures.
SOLUTIONS
- a) History from the patient or the on lookers.
- Pain aggravated by movement
- Tenderness over the fractured limb
- Loss of function of the affected part or the whole limb
- Deformity
- Shortening of the limb
- Abnormal mobility at the affected area
- Creepers or grating of the bone ends as they move each other
- Swelling of the affected part
- Shock may occur
- The bone may be seen out if it’s a compound fracture
b)
- Tissue fragments between bone ends; Splinters of dead bone (sequestrate) and soft tissue fragments not removed by phagocytosis delay healing.
- Deficient blood supply; this delays growth of granulation tissue and new blood vessels. Hypoxia also reduces the number of osteoblasts and increases the number of chondrocytes that develop from there common parent cells. This may lead to cartilaginous union of fracture which results in a weaker repair.
- Poor alignment of bone ends: This may result in the formation of a callus that heals slowly and often results in permanent disability
- Continued mobility of bone ends; Continuous movement results in fibrosis of the granulation tissue followed fibrous union of the fracture.
- Miscellaneous; this include
- Infection; pathogens enter through broken skin, although they occasionally be blood borne, healing will not occur until infection resolves
- System illness
- Malnutrition
- Drugs e.g. Corticosteroids
- Aging
c)
- Following a fracture the broken ends of a bone a joined by the deposition of a new bone. This occurs in several stages
- Hematoma forms between the ends of the bone and in the surrounding soft tissues.
- There follows development of acute inflammation and accumulation of inflammatory exudates, continuing microphages that phagocytosis the hematoma and small fragments of a bone without blood supply(this takes place about five days). Fibroblasts migrate to the site, granulation tissue and the new capillaries develop.
- New bone forms as large numbers of osteoblasts secretes spongy bone, which unit the broken ends, and is protected by the outer layer of the bone and cartilage, this new deposits of bone and cartilage are called callus.
- Over the next few weeks, the callus matures and the cartilage is gradually replaced by new bone
- Reshaping of the bone continues and gradually the medullary canal is re –opened through the callus (in weeks or month). In time the bone heals completely with callus tissue replaced with mature compact bone. Often the bone is thicker and stronger at the repair site that originally, and the second is more likely to occur at a different site.
- d) Complications of fractures are divided in to two.
- General complications.
- Local complications
- General complications are;
- Hemorrhage which may lead in to shock.
- Fat embolism
- Infections
- Hypostatic Pneumonia
- Damage to the nearby structures
- Local complications
- Keloids
- Loss of function
- Damage to the nerves
- Necrosis
- Delayed union of bones; this may be as a result of incomplete reduction, inadequate immobilization, lack of blood supply to areas, infection which disrupt formation
- Malunion of the bones; this when there’s failure of bone fragments to unit. This as a result of a big gap between the fragment











 electroencephalogram
electroencephalogram