Epilepsy
A seizure is an occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain. Epilepsy, however, is a chronic disorder characterized by recurrent, unprovoked seizures.
Epilepsy is a neurological disorder in which the brain activity becomes abnormal, causing seizures or periods of unusual behaviour, sensations, and sometimes loss of awareness.
The modern definition, established by the International League Against Epilepsy (ILAE), provides clear criteria for diagnosis.
Epilepsy Definition (ILAE)
Epilepsy is defined by the International League Against Epilepsy (ILAE) as a disease of the brain defined by any of the following conditions:
- At least two unprovoked (or reflex) seizures occurring more than 24 hours apart.
- Unprovoked Seizures: These are seizures that occur without any immediate identifiable cause. This differentiates them from "provoked" seizures, which are acute symptomatic seizures triggered by a temporary or reversible systemic or brain insult (e.g., severe electrolyte imbalance, acute stroke, drug intoxication/withdrawal, high fever in children). A single provoked seizure does not typically lead to a diagnosis of epilepsy.
- Reflex Seizures: These are seizures reliably induced by a specific afferent stimulus or specific cognitive activity (e.g., photosensitive epilepsy where seizures are triggered by flashing lights). While provoked, if they recur, they fall under the definition of epilepsy.
- One unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years.
- This criterion acknowledges that some individuals, after a single unprovoked seizure, have underlying conditions (e.g., an epileptogenic lesion on MRI, certain abnormal EEG findings) that confer a high risk of recurrence, essentially making the epilepsy diagnosis certain even after one event. Examples include:
- An individual with a clear structural lesion in the brain (e.g., old stroke, tumor, malformation).
- Specific epileptiform abnormalities on EEG.
- Certain genetic syndromes.
Differentiating Epilepsy from a Single Seizure:
- Single Seizure: A person can have one seizure without having epilepsy. This could be a provoked seizure (e.g., due to acute illness, drug overdose, high fever) or a single unprovoked seizure where the risk of recurrence is low (less than 60%). Many individuals will never have another seizure after a first unprovoked event.
- Epilepsy: Implies a predisposition to generate seizures due to an underlying chronic brain disorder, requiring ongoing management.
Resolution of Epilepsy:
The ILAE also provides criteria for when epilepsy can be considered "resolved" for practical clinical and epidemiological purposes:
- Individuals who have been seizure-free for 10 years, with no anti-seizure medication for the last 5 years.
- Individuals who have reached the age-dependent remission criteria for an epilepsy syndrome that is known to resolve with age (e.g., benign epilepsy with centrotemporal spikes, childhood absence epilepsy).
Etiology of Epilepsy (The Cause)
The cause of epilepsy can be identified in many cases, though for some, the cause remains unknown. The International League Against Epilepsy (ILAE) classifies the etiologies of epilepsy into six main categories:
- Structural: Epilepsy caused by a visible abnormality in the brain structure. These abnormalities can be seen on imaging scans (like MRI).
- Examples:
- Brain tumors: Abnormal growths in the brain.
- Stroke: Damage to the brain due to interruption of its blood supply.
- Traumatic Brain Injury (TBI): Head trauma from accidents, falls, domestic violence, or other impacts. This includes both acute injury and the resulting scar tissue.
- Brain malformations: Abnormal development of the brain before birth (e.g., cortical dysplasia).
- Scar tissue: Specifically, scar tissue in areas like the temporal lobe (often from previous injury, infection, or stroke) can create an epileptic focus.
- Prior hypoxia/anoxia: Brain damage due to lack of oxygen (e.g., at birth, or from other medical events).
- Genetic: Epilepsy caused by a known or presumed genetic mutation. These can be inherited or occur spontaneously.
- Examples:
- Familial epilepsy: Conditions that clearly run in families, suggesting an inherited genetic predisposition.
- Specific genetic syndromes: Many syndromes are now known to involve epilepsy as a symptom.
- Infectious: Epilepsy resulting from a central nervous system (CNS) infection that causes brain inflammation or damage.
- Examples:
- Meningitis: Inflammation of the membranes surrounding the brain and spinal cord.
- Encephalitis: Inflammation of the brain itself.
- AIDS/HIV: The virus or opportunistic infections associated with it can damage the brain.
- Neurocysticercosis: Parasitic infection affecting the brain.
- Metabolic: Epilepsy due to an underlying metabolic disorder that disrupts the brain's normal chemical balance and function.
- Examples:
- Inborn errors of metabolism: Genetic disorders that affect the body's ability to process nutrients (e.g., Phenylketonuria, mitochondrial disorders).
- Electrolyte imbalances: Severe disturbances in sodium, calcium, magnesium levels.
- Hypoglycemia/Hyperglycemia: Critically low or high blood sugar levels.
- Immune: Epilepsy caused by an autoimmune process where the body's immune system mistakenly attacks healthy brain cells.
- Examples:
- Autoimmune encephalitis: Inflammation of the brain caused by antibodies attacking brain proteins (e.g., anti-LGI1, anti-NMDA receptor encephalitis).
- Systemic autoimmune diseases: Lupus, celiac disease, etc., can sometimes be associated with epilepsy.
- Unknown: When, despite thorough investigation, the cause of the epilepsy cannot be identified. This category applies when there's insufficient evidence to place it in one of the other categories.

Major Types of Epilepsy and Seizures
Epilepsy and seizures are broadly categorized based on where the seizure activity begins in the brain. Here, we'll explore some common types, including generalized seizures (affecting both sides of the brain) and focal seizures (starting in one area).
1. Generalized Tonic-Clonic Seizures (Formerly "Grand Mal Epilepsy")
Generalized tonic-clonic seizures are a major form of epilepsy characterized by a total loss of consciousness and a dramatic, convulsive event. These seizures typically last between 3 to 5 minutes. Following the seizure, the individual spontaneously regains consciousness but may experience confusion or injury sustained during the episode.
A generalized tonic-clonic seizure occurs in four distinct phases:
Aura Phase (Pre-seizure Warning):
- Occurs in approximately 50% of patients and lasts less than 10 seconds.
- This is a brief warning sensation that can include: Unusual sounds or flashes of light, a peculiar taste in the mouth, feelings of weakness, dizziness, or numbness in a limb, or a brief stomach pain.
Tonic Phase (Stiffening):
- If an aura is present, this phase follows immediately.
- Characterized by a complete loss of consciousness and falling.
- All muscles contract, causing the body to become rigid and hyperextended.
- Often accompanied by a cry as air is forcefully expelled through tightened vocal cords.
- This phase typically lasts for about 20 seconds.
Clonic Phase (Jerking):
- Follows the tonic phase.
- Involves repeated, rhythmic contractions and relaxations of all body muscles.
- Results in gross motor activity, including jerking of the limbs.
- During this phase, bladder control may be lost, and in rare cases, bowel control.
- Frothy saliva may come from the mouth; it can be blood-stained if the tongue or lips were bitten.
- This phase lasts between 30 to 90 seconds.
Deep Sleep / Post-convulsive Phase (Post-ictal Period):
- The individual enters a deep sleep that can last for up to two hours.
- Upon waking, confusion and disorientation are common for several minutes.
- Headache is a frequent complaint.
- Amnesia for the entire seizure event is typical.
2. Absence Seizures (Formerly "Petit Mal Epilepsy")
Absence seizures are a minor form of epilepsy, commonly occurring in children. They are often mistaken for daydreaming due to their subtle nature and lack of a dramatic convulsion or fall.
- Brief Loss of Awareness: A sudden, brief interruption of consciousness, typically lasting 15 seconds or less.
- Staring Spells: The person will typically stop moving and stare blankly in one direction.
- No Fall: The individual usually does not fall down and is often unaware that a seizure has occurred.
- Subtle Movements: Slight muscle contractions may occur, but bladder control is rarely lost.
- Rapid Recovery: Normal alertness returns immediately after the episode, though the person may not recall the event.
- Childhood Onset: Often begins in childhood and may resolve during adolescence, or in some cases, evolve into other seizure types.
- Impact on Daily Life: Frequent episodes can lead to poor academic performance or appear as a child dropping objects unknowingly.
3. Atonic Seizures (Drop Attacks)
Atonic seizures are characterized by a sudden and complete loss of muscle tone.
- Sudden Muscle Relaxation: The body goes limp, causing the person to slump or collapse.
- Risk of Injury: The sudden fall can lead to significant injury.
- Associated Syndromes: Atonic seizures are a hallmark of certain epilepsy syndromes, such as Lennox-Gastaut syndrome.
4. Myoclonic Seizures
Myoclonic seizures involve sudden, brief, shock-like muscle jerks or increases in muscle tone.
- Sudden "Jolts": The person experiences abrupt, involuntary jerks, similar to those sometimes felt when falling asleep (sleep myoclonus).
- Repetitive Nature: Myoclonic seizures can occur in bouts, potentially causing harm if they lead to falls or dropped objects.
Infantile Spasms (A Subtype of Myoclonic Epilepsy)
- Onset: Typically begins between 3 and 12 months of age and can persist for several years.
- Presentation: Consist of a sudden jerk followed by stiffening. Often, the child's arms fling outward as the knees pull up and the body bends forward.
- Duration: Each spasm lasts only a second or two but usually occurs in a series, close together.
- Misdiagnosis: Sometimes mistaken for colic, but colic cramps do not typically occur in a series.
- Timing: Most common just after waking up or falling asleep.
- Severity: This is a particularly severe form of epilepsy that requires prompt evaluation and treatment due to its potential lasting effects on child development.
5. Focal Seizures (Starting in One Area)
Focal seizures, previously known as partial seizures, originate in a specific area of the brain. The symptoms depend entirely on the brain region affected.
a. Jacksonian Epilepsy (Motor Focal Seizures)
Jacksonian epilepsy refers to a type of focal seizure that begins in the motor sensory area of the cerebral cortex.
- Localized Onset: Disrupts the function of a particular body part due to excessive electrical discharges from a focal point in the brain.
- "March" of Symptoms: Symptoms may begin in a small area (e.g., twitching in a thumb or finger) and then gradually spread to involve an entire limb or even the whole side of the body.
- Secondary Generalization: The seizure can insidiously or gradually spread to become a generalized tonic-clonic seizure.
b. Temporal Lobe Epilepsy (Focal Seizures with Impaired Awareness)
Temporal lobe seizures begin in the temporal lobes, which are critical for processing emotions, memory, and language. These lobes are vulnerable to conditions like anoxia at birth, anatomical defects, or scarring.
- Variable Awareness: The patient may remain partially aware during some temporal lobe seizures. However, in more intense seizures, the individual might appear awake but be unresponsive, displaying repetitive, purposeless movements.
- Automatisms: Common automatisms (repetitive movements) include: Chewing, Swallowing, Lip smacking, Unusual finger movements (e.g., picking motions).
- Emotional and Sensory Symptoms: Symptoms can be related to the temporal lobe's functions, leading to:
- Odd feelings like euphoria, déjà vu (a feeling of having experienced something before), or fear.
- A sudden, strange odor or taste.
- A rising sensation in the abdomen.
- Aura (Warning Sensation):
- An unusual sensation, or aura, often precedes a temporal lobe seizure, acting as a warning. Not everyone experiences or remembers auras.
- The aura is the initial part of the focal seizure before consciousness is significantly impaired.
- Examples include: a sudden sense of unprovoked fear or joy, déjà vu, or a strange smell/taste.
- Duration: Typically lasts 30 seconds to two minutes for seizures with impaired awareness.
- Post-seizure (Post-ictal) Period: After a temporal lobe seizure, the patient may experience: A period of confusion and difficulty speaking, Inability to recall what occurred during the seizure, Unawareness of having had a seizure, Extreme sleepiness.
- Potential for Generalization: In some cases, a temporal lobe seizure can evolve into a generalized tonic-clonic seizure.
- Treatment: Primarily treated with medication. For individuals unresponsive to medication, surgery may be an option.
Key Terminology:
- Tonic: Refers to stiffening of the muscles.
- Clonic: Refers to jerking of the muscles.
- Tonic-Clonic: Involves both stiffening followed by jerking.
- Atonic: Characterized by a loss of muscle tone, causing the body to go limp.
- Myoclonic: Involves recurrent, brief jerks of a body part.
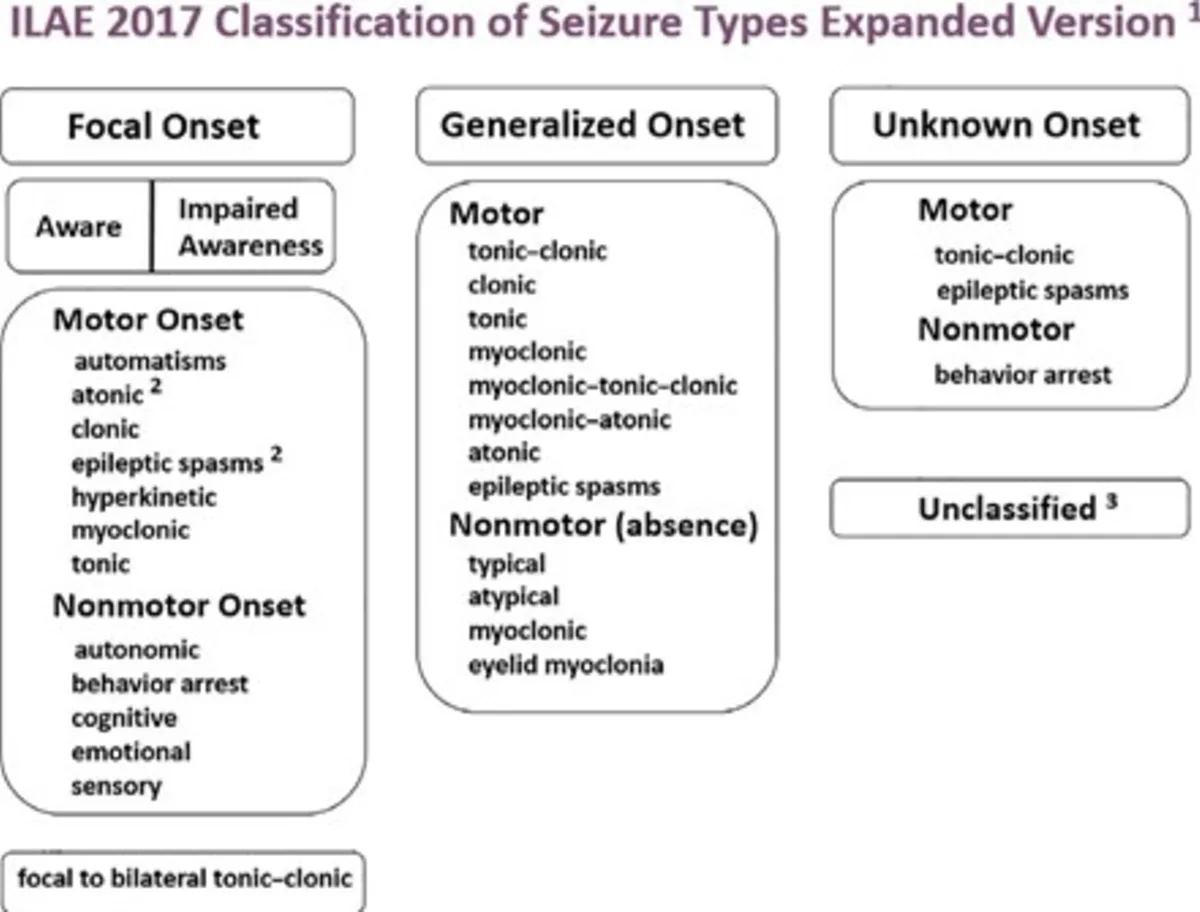
ILAE Classification: Seizure and Epilepsy Types
The International League Against Epilepsy (ILAE) classification provides a detailed framework for understanding seizures (the event) and epilepsy (the underlying condition).
Table 1: Classification of Seizure Types (The Event)
| Seizure Type Category |
Subtype |
Key Characteristics & Observable Features |
Correlation with Previous Notes |
| I. Focal Onset Seizures |
Focal Aware Seizure |
- Originates in one area/hemisphere of the brain.
- Consciousness preserved throughout.
- "Aura" is now understood as a Focal Aware Seizure with specific sensory, emotional, cognitive, or autonomic symptoms (e.g., peculiar taste, dizziness, abdominal rising sensation, déjà vu).
|
Directly correlates with "Aura phase" from Grand Mal and early symptoms of Temporal Lobe/Jacksonian seizures. |
|
Focal Impaired Awareness Seizure |
- Originates in one area/hemisphere of the brain.
- Consciousness is impaired at any point (dazed, confused, unresponsive).
- Motor Features: Automatisms (e.g., chewing, lip smacking, picking motions), atonic (localized limpness), clonic (localized jerking), epileptic spasms, hyperkinetic (fidgeting, thrashing), myoclonic (localized jerks), tonic (localized stiffening).
- Non-Motor Features: Autonomic (e.g., heart rate changes), behavioral arrest, cognitive (e.g., difficulty speaking), emotional (e.g., unprovoked fear or joy), sensory experiences (e.g., strange smell/taste).
|
Correlates with Temporal Lobe Epilepsy (purposeless repetitive movements, unresponsiveness) and Jacksonian Epilepsy (localized twitching/tremors). |
|
Focal to Bilateral Tonic-Clonic Seizure |
- A focal seizure (aware or impaired awareness) that then spreads to involve both hemispheres, leading to a generalized tonic-clonic event.
- Includes the Tonic phase (sustained stiffening, falling, cry), Clonic phase (rhythmic jerking, potential bladder/bowel release, frothy/blood-stained saliva), followed by a Post-ictal phase.
|
Corresponds to what was previously often described as "Grand Mal Epilepsy" particularly if it began with an "aura." |
| II. Generalized Onset Seizures |
Tonic-Clonic |
- Originates rapidly in both hemispheres from the outset.
- Consciousness typically impaired immediately.
- Classic sequence: Tonic phase (total body stiffening, loss of consciousness, fall, epileptic cry) followed by Clonic phase (repeated, rhythmic jerking of all muscles, potential incontinence, tongue/lip biting), ending in a Post-ictal phase (deep sleep, confusion, headache, amnesia).
|
Corresponds to "Grand Mal Epilepsy" (Generalized Tonic-Clonic Epilepsy) when there's no preceding focal onset/aura. |
|
Tonic |
- Sustained stiffening of muscles throughout the body, without a subsequent clonic phase.
- Consciousness typically impaired.
|
Relates to the stiffening aspect of "Tonic and Clonic Seizures." |
|
Clonic |
- Rhythmic jerking movements of muscles throughout the body, without a preceding tonic phase.
- Consciousness typically impaired.
|
Relates to the jerking aspect of "Tonic and Clonic Seizures." |
|
Atonic |
- Sudden, generalized loss of muscle tone; body goes limp, slump or collapse ("drop attacks").
- Consciousness typically impaired.
|
Directly correlates with Atonic Seizures (Drop Attacks). |
|
Myoclonic |
- Brief, shock-like jerks or increases in muscle tone, affecting muscles or muscle groups.
- Can occur in bouts.
|
Directly correlates with Myoclonic Seizures. |
|
Epileptic Spasms |
- Sudden flexion or extension of the body (e.g., arms fling outward, knees pull up, body bends forward).
- Often occur in clusters.
|
Directly correlates with Infantile Spasms. |
|
Typical Absence |
- Brief (seconds) staring spells with unresponsiveness.
- Often mistaken for daydreaming.
- May involve subtle automatisms.
- Consciousness impaired.
|
Directly correlates with "Petit Mal Epilepsy" (Absence Seizures). |
|
Other Absence Types |
Atypical Absence, Myoclonic Absence, Eyelid Myoclonia (more specific subtypes). |
|
| III. Unknown Onset Seizures |
- |
- When the beginning of the seizure is not observed or cannot be determined.
- May later be reclassified once more information is available.
|
Applies when the initial moments of an event (e.g., a Tonic-clonic seizure or Epileptic spasm) are unwitnessed. |
Table 2: Pre-Seizure and Post-Seizure Stages
| Stage |
Characteristics |
| Prodrome |
- Non-specific symptoms occurring hours or days before a seizure.
- Not part of the seizure activity itself.
- Examples: Mood changes (irritability, depression), talkativeness, restlessness, violence.
|
| Post-ictal Stage |
- The period immediately after a seizure as the brain recovers.
- Symptoms vary based on seizure type and intensity.
- Examples: Confusion, fatigue, headache, amnesia for the event, disorientation, emotional changes (calmness, quietness, isolation, retarded mobility, depression).
|
Clinical Manifestations (What Epilepsy Looks Like)
Clinical manifestations are the signs and symptoms that occur during a seizure event and can be highly varied, depending on the seizure type and the brain region involved.
1. Generalized Onset Seizures:
Generalized Tonic-Clonic Seizure (formerly Grand Mal):
- Prodrome (Pre-ictal): Hours or days before the seizure, the person may experience non-specific symptoms like mood changes, irritability, or difficulty concentrating.
- Aura (often absent or not remembered if truly generalized onset): If present, it would indicate a focal onset that rapidly generalized.
- Tonic Phase: Sudden loss of consciousness, body stiffens symmetrically, often a cry or groan (as air is forced out). Person falls to the ground. Eyes roll back. Breathing may stop briefly, leading to cyanosis. Lasts usually 10-30 seconds.
- Clonic Phase: Rhythmic jerking of the limbs and body, typically bilateral. May involve tongue biting (often side of tongue), incontinence (bladder and rarely bowel), frothing at the mouth (which can be blood-stained from biting). Lasts usually 30 seconds to 2 minutes.
- Post-ictal Phase: Gradual recovery of consciousness. Confusion, drowsiness, headache, muscle aches, and complete amnesia for the event are typical. May enter a deep sleep. Can last minutes to hours.
Absence Seizures (formerly Petit Mal):
- Onset: Typically abrupt, without warning.
- Manifestations: Brief (seconds, typically <15-20 sec) episodes of staring, blank expression, unresponsiveness. May involve subtle automatisms like eyelid fluttering, lip-smacking, or mild head nodding.
- Termination: Abrupt. The individual quickly resumes prior activity, often unaware of the seizure or with immediate return of alertness. No post-ictal confusion.
- Typical Population: Most common in children, often mistaken for daydreaming or inattention.
Myoclonic Seizures: Sudden, brief, shock-like jerks or twitches of a muscle or group of muscles (e.g., arms, shoulders, head). Often bilateral but can be unilateral. Consciousness usually preserved.
Atonic Seizures (Drop Attacks): Sudden, brief loss of muscle tone, causing the person to fall abruptly to the ground, often without warning. High risk of head and facial injury.
Tonic Seizures: Sudden, brief stiffening or tensing of muscles, typically in the trunk and limbs. Can cause falls.
Clonic Seizures: Rhythmic jerking movements, usually symmetrical, but without the preceding tonic phase.
2. Focal Onset Seizures:
Focal Aware Seizures (formerly Simple Partial):
- Manifestations: Vary widely depending on the brain region affected, but consciousness is fully preserved. The person is aware of the event.
- Motor: Twitching, jerking, or stiffening of a specific body part (e.g., face, arm, leg). "Jacksonian March" describes the spread of motor symptoms.
- Sensory: Tingling, numbness, visual disturbances, auditory hallucinations, olfactory, gustatory, or vertigo.
- Autonomic: Changes in heart rate, breathing, sweating, epigastric rising sensation, flushing, pallor.
- Psychic/Cognitive/Emotional: Feelings of fear, anxiety, déjà vu, jamais vu, memory disturbances. Often experienced as an "aura" before evolving to a more complex seizure.
Focal Impaired Awareness Seizures (formerly Complex Partial):
- Manifestations: Consciousness is impaired or lost. The person may appear to be awake but is unresponsive, confused, or has an altered state of awareness.
- Automatisms: Repetitive, non-purposeful movements are common (e.g., lip-smacking, chewing, swallowing, fumbling with clothes, walking aimlessly, repeating phrases). These are characteristic of temporal lobe seizures.
- Duration: Typically 30 seconds to 2 minutes.
- Post-ictal Phase: Common, characterized by confusion, drowsiness, and often amnesia for the seizure event.
Focal to Bilateral Tonic-Clonic Seizure: Begins with symptoms of a focal seizure (e.g., an aura, focal motor activity, or impaired awareness), then rapidly progresses to a generalized tonic-clonic seizure with loss of consciousness.
Diagnosis of Epilepsy
Diagnosing epilepsy involves confirming that the events are indeed epileptic seizures, classifying the seizure type, identifying the epilepsy syndrome, and determining the etiology.
1. Clinical History (The Most Crucial Step):
- Detailed Seizure Description: A meticulous history from the patient (if possible) and, crucially, from an eyewitness (family member, friend, colleague) is paramount. Questions focus on:
- Pre-event: Prodrome, triggers, warning signs (aura).
- During the Event: Onset (sudden vs. gradual), movements (type, location, symmetry), vocalizations, eye movements, head turning, color changes, incontinence, tongue biting, level of awareness/responsiveness.
- Post-event: Duration of confusion, memory of the event, fatigue, headache, muscle soreness.
- Medical History: Birth history, developmental milestones, head injuries, CNS infections, fevers, family history of epilepsy, past medical conditions, medications, drug/alcohol use.
2. Neurological Examination:
- Usually normal between seizures, but may reveal focal deficits if there is an underlying brain lesion (e.g., hemiparesis, sensory loss). Post-ictally, transient neurological deficits (Todd's paralysis) can be observed.
3. Electroencephalography (EEG):
- Purpose: Records the electrical activity of the brain to identify abnormal brain wave patterns (epileptiform discharges).
- Interictal EEG: Performed between seizures. Can show characteristic patterns (e.g., spikes, sharp waves) that support a diagnosis. A normal interictal EEG does not rule out epilepsy.
- Ictal EEG: Performed during a seizure (e.g., during video-EEG monitoring). Captures the actual seizure activity and is the most definitive EEG finding for diagnosis and localization.
- Activation Procedures: Hyperventilation, photic stimulation, and sleep deprivation are used to provoke epileptiform activity.
4. Neuroimaging:
- Magnetic Resonance Imaging (MRI) of the Brain:
- Purpose: To identify structural abnormalities causing seizures (e.g., tumors, strokes, malformations, mesial temporal sclerosis).
- Importance: Crucial for identifying the etiology, especially in focal epilepsies.
- Computed Tomography (CT) Scan of the Brain: Less sensitive than MRI but can be used in emergency situations (e.g., to rule out acute hemorrhage).
5. Blood Tests and Other Laboratory Investigations:
- To rule out other conditions that can cause seizures (e.g., metabolic derangements, infections, drug/alcohol withdrawal, electrolyte imbalances). Examples: CBC, electrolytes, glucose, liver/kidney function tests, toxicology screen.
6. Video-EEG Monitoring:
- Continuous simultaneous recording of EEG and video of the patient over several days. Gold standard for confirming diagnosis, classifying seizure types, and localizing onset zone for surgery.
Management and Treatment Options for Epilepsy
The management of epilepsy is multifaceted, encompassing immediate care during a seizure, long-term pharmacological and non-pharmacological treatments, addressing complications, and providing comprehensive patient education.
I. Immediate Management and First Aid During a Seizure (Emergency Management):
A seizure can be frightening for bystanders, but knowing how to act can prevent injury and ensure patient safety.
1. General Principles of Emergency Management:
- Stay Calm: Remain composed and speak calmly.
- Safety First: Remove the person from immediate danger (e.g., clear sharp objects). If the patient is safe, do not move them.
- Time the Seizure: Note the exact start time. Crucial for determining if medical help is needed.
- Loosen Clothing: Around the neck to ease breathing.
- Protect the Head: Support with a soft, flat material (e.g., folded jacket).
- Ensure Airflow: Clear space and minimize crowds.
- Recovery Position: As soon as jerking stops, turn onto side to prevent choking.
- Check Breathing: If breathing sounds difficult after the seizure, call for an ambulance.
- Clear Airway: Gently check for blocks (e.g., false teeth) but do not force mouth open.
- Stay with Patient: Until fully awake and reoriented.
- Reassurance: Reorient and reassure the patient after recovery.
2. What NOT to Do During a Seizure:
- Do not put any hard object (e.g., spoon) in the person's mouth.
- Do not hold their limbs tightly.
- Do not give anything to eat or drink until fully alert.
- Do not attempt mouth-to-mouth resuscitation (unless breathing doesn't resume after seizure).
3. When to Call for Emergency Medical Help:
- The person has never had a seizure before.
- The person has difficulty breathing or waking up after the seizure.
- The seizure lasts longer than 5 minutes (Potential Status Epilepticus).
- The person has another seizure soon after the first one without full recovery.
- The person is hurt during the seizure.
- The seizure happens in water.
- The person has a pre-existing health condition like diabetes, heart disease, or is pregnant.
II. Long-Term Medical Management (Drug Management):
The cornerstone of long-term epilepsy treatment is typically anti-seizure medications (ASMs).
1. Principles of Pharmacological Treatment:
- Goal: Reduce frequency of seizures or eradicate them.
- Individualized Treatment: Based on seizure type, age, comorbidities.
- Titration: Start low and gradually increase.
- Monitoring: Regular follow-ups for progress and side effects.
- Monotherapy vs. Polytherapy: Start with one drug; add others if needed.
2. Commonly Used Anti-Seizure Medications:
- Phenobarbitone: Typically 30 to 90 mg two to three times daily (divided doses). An older, broad-spectrum ASM.
- Phenytoin Sodium: Typically 100-300 mg daily (DDD - once daily or divided doses). Effective for focal and generalized tonic-clonic seizures.
- Sodium Valproate (Valproic Acid): Typically 200-1200 mg two to three times daily (divided doses). Broad-spectrum, effective for various seizure types.
- Carbamazepine: Typically 100-1200 mg in 3 divided doses. Primarily used for focal seizures.
3. General Principles of the Treatment of Epilepsy:
- Treat Causative Factors: Treat underlying causes like malaria, meningitis, or cerebral growths.
- Avoidance of Precipitating Factors: Identify and avoid triggers.
- Anticipation of Natural Variation: Understand seizure timing (e.g., during sleep).
- Appropriate and Regular Administration: Strict adherence to prescribed regimen.
Seizure Triggers & Complications
Seizure Triggers:
- Physiological Stressors: Fevers, sleep deprivation, fasting.
- Emotional Stressors: Fear, anger, excitement.
- Sensory Stimuli: Flickering lights (photosensitivity), specific sounds.
- Substance Use: Alcohol intoxication or withdrawal.
- Environmental Factors: Fatigue, boredom, high altitude.
- Hormonal Changes: Menstrual cycle fluctuations.
- Medication Non-adherence.
Complications of Epilepsy:
- Status Epilepticus: A medical emergency defined by a seizure lasting longer than 5 minutes, or recurrent seizures without return to baseline consciousness. Requires urgent medical treatment.
- Mental Deterioration (Cognitive Impairment): Chronic brain syndrome where repeated seizures can lead to progressive brain damage.
- Physical Injuries: Falls, burns, fractures.
- Psychosocial Issues: Stigma, anxiety, depression, social isolation.
- SUDEP (Sudden Unexpected Death in Epilepsy): The most common cause of epilepsy-related death where no other cause is found.
Patient and Community Education & Prevention
For Patients and Caretakers:
- Epilepsy is an illness like any other; with treatment, a person can lead a full life.
- Encourage participation in activities safely.
- Emphasize importance of taking medications exactly as prescribed.
- Advise against dangerous activities (swimming alone, driving until seizure-free, operating heavy machinery).
For the Community:
- Combat Stigma: Educate that labeling patients is traumatizing.
- Inclusion: Children should attend school; adults should be encouraged to marry.
- Contagion: Teach that epilepsy is not contagious.
Prevention of Epilepsy:
- Prevent Head Injury (seat belts, helmets).
- Seek Immediate Medical Attention after a first seizure.
- Good Prenatal Care.
- Manage Cardiovascular Risk Factors (hypertension).
- Avoid Excess Alcohol Abuse.
- Manage Fevers in Children.
- Treat Infections and Ensure Nutrition.
Nursing Diagnoses and Specific Nursing Interventions
Nursing Diagnosis 1: Risk for Injury
Related to uncontrolled seizure activity, loss of consciousness, uncontrolled muscle movements, or falls during seizures.
| Specific Nursing Interventions |
Details |
| Seizure Precautions |
Pad side rails, keep bed in lowest position, instruct to avoid sharp objects in environment, recommend medical alert bracelet. |
| Seizure First Aid |
Stay with patient, protect head, loosen clothing, turn to recovery position, move furniture, DO NOT restrain or insert objects in mouth, time the seizure. |
| Post-Ictal Monitoring |
Monitor vital signs, level of consciousness, assess for injuries, allow rest, reorient gently. |
| Activity Modification |
Educate on avoiding swimming alone, driving restrictions, and home modifications (e.g., shower chair). |
Nursing Diagnosis 2: Ineffective Airway Clearance
Related to neuromuscular impairment during tonic-clonic seizures (tongue biting, increased salivary secretions, aspiration risk).
| Specific Nursing Interventions |
Details |
| Acute Seizure Management |
Do not attempt to open mouth during seizure. Once movements cease, turn to recovery position to facilitate drainage. Suction secretions as needed. |
| Post-Ictal Assessment |
Monitor respiratory rate/depth, assess breath sounds for aspiration (crackles), monitor for hypoxia. |
| Patient Education |
Educate family on recovery position importance and when to call for emergency help if breathing is compromised. |
Nursing Diagnosis 3: Inadequate Health Knowledge
Regarding epilepsy (disease process, triggers, medication regimen, first aid, emergency protocols).
| Specific Nursing Interventions |
Details |
| Comprehensive Education |
Explain disease process, ASM purpose/dose/side effects, importance of daily dosing, triggers, first aid, and emergency protocols (e.g., seizure >5 min). |
| Resources |
Provide written materials and support group info. |
| Teach-Back & Reinforcement |
Ask patient to explain back what they learned; reinforce at every visit. |
Nursing Diagnosis 4: Excessive Anxiety
Related to unpredictable nature of seizures, fear of public seizures, social stigma.
| Specific Nursing Interventions |
Details |
| Establish Trust |
Provide non-judgmental environment to express fears. |
| Empowerment |
Address knowledge deficits to reduce anxiety; emphasize productive lives are possible. |
| Coping Strategies |
Encourage relaxation techniques, mindfulness, support group participation. |
| Referrals & Stigma |
Refer to mental health professionals if needed; discuss strategies for talking to employers/friends. |
Nursing Diagnosis 5: Impaired Social Interaction
Related to fear of seizures in public, stigma, withdrawal, or limitations on activities.
| Specific Nursing Interventions |
Details |
| Address Anxiety/Deficits |
Educate to dispel myths; help patient develop confidence in disclosing condition. |
| Promote Participation |
Discuss safe activities (e.g., cycling with supervision), public transport options, encourage social groups. |
| Support & Advocacy |
Recommend support groups; advocate for patient in social settings by educating others on first aid. |
Nursing Diagnosis 6: Noncompliance (Medication Adherence)
Related to perceived side effects, forgetfulness, or lack of understanding.
| Specific Nursing Interventions |
Details |
| Detailed Teaching |
Explain "why" consistent use prevents complications. Review side effects and strategies to manage them. |
| Adherence Strategies |
Suggest pill organizers, alarms, linking to daily routines. Discuss refilling prescriptions early. |
| Address Beliefs |
Explore beliefs/misconceptions. Involve family in medication management if appropriate. |
Nursing Diagnosis 7: Fatigue
Related to post-ictal state, sleep disturbance, or side effects of anti-seizure medications.
| Specific Nursing Interventions |
Details |
| Assessment & Sleep Hygiene |
Assess fatigue severity. Educate on regular sleep schedules, avoiding caffeine/alcohol before bed. |
| Medication Review |
Collaborate with physician if ASMs cause excessive sedation. |
| Energy Conservation |
Teach pacing activities and prioritizing tasks. |
| Healthy Lifestyle |
Encourage regular exercise and balanced diet. Allow adequate rest post-seizure. |